Prevention & Treatment
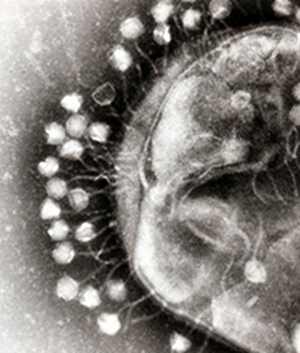
Bacteriophage products
Brimrose Technology Corporation is working directly with the Eliava Institute in Tblisi, Georgia to offer bacteriophage products to customers. The Eliava Institute provides the best bacteriophage products in the world and is proud of its rich, nearly 100 year history.
Bacteriophage products are highly valued viruses that are able to devour bad bacteria that cause sickness and death and thus are of great importance in animal feed products. Their use is gaining great momentum as they are seen as being a natural alternative to antibiotics.
Phage Products
Pyo-Phage (5 components: Staphylococcus, E. coli, Streptococcus, Pseudomonas, Proteus)
Intesti Phage (17 components: multiple phage components - Shigella spp., Salmonella spp., Cholera suis, Staphylococcus, Proteus spp., E.coli – different serotypes, P.aeruginosa)
SES Phage (Staphylococcus phage, Streptococcus phage, E.coli)
EnkoPhagum (Salmonellae [Paratyphus A, Paratyphus B, Typhimurium, Enteritidus, Choleraesuis, Oranienburg, Dublin, Anatum], Shigellas [Flexner, Zonne], Enterepathogenic serotypes of Escherichia coli [10 types], Staphylococcus [3 types])
Fersisi Phage (Staphylococcus phage [3 types] Streptococcus phage [4 types])
Mono-phage Preparations (Staphylococcal, E. coli, Streptococcal, Pseudomonas aeruginosa, Proteus)
For more information contact:
Dr. Yingyun Liu
Brimrose Technology Corporation
410-472-2600
Therapeutic Use Antibodies
The BTC Team, working with partners in the Former Soviet Union (FSU), USA and Singapore, has been collaborating on the discovery and development of antibodies for therapeutic use. This includes specific anti-cytokine antibodies and Anthrax as well as generic platform antibodies.
Controlling The Inflammatory Response - Interferon-Gamma (IFNγ)
The application of interferon-gamma (IFNγ) is based on the pioneering work of Simon Skurkovich, MD, PhD who first proposed in the journal Nature* that down-regulating specific cytokine activity in autoimmune disease would be beneficial in autoimmune disease. Cytokines are a class of immunoregulatory substances that are secreted by cells of the immune system to protect the body against disease. The principal focus has been antibodies to IFN-γ for the treatment of these and other Th1 autoimmune diseases. T helper (Th) cells differentiate under certain circumstances into two distinct subsets: Th1 and Th2 cells. The functional differences are explained primarily through the activities of the cytokines they secrete. IFN-g is the signature Th1 cytokine. If Th1 cells are over-produced, there is an imbalance or polarization toward Th1 cell-produced cytokines, often called inflammatory cytokines. Chronic production, particularly of IFN-g, can lead to autoimmune diseases. IFN-g can trigger a chain of other immune system substances that lead to a loss of homeostasis (a relatively stable state of equilibrium) and pathology (structural and functional deviations from the normal that constitute disease or characterize a particular disease). **
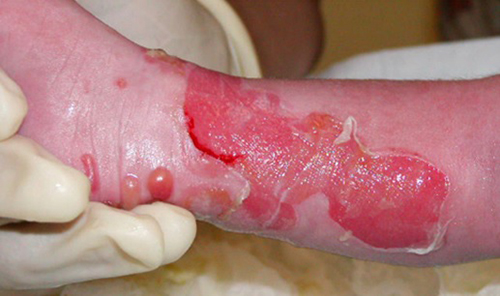
During laboratory studies focused on skin related issues, it was discovered that interferon-gamma (IFNγ) directly promotes the aggregation of mutant keratin proteins. IFNγ also rendered organotypic cultures more fragile, reduced cell growth and delayed wound healing. Humanized monoclonal antibodies to IFNγ neutralized the biological activity as it prevented IFNγ-mediated keratin aggregation, restored a normal cell growth and a faster wound healing despite the presence of IFNγ. These results provide new insights on the effect of inflammation and IFNγ on keratinocytes. This suggest potential applications for the treatment of various skin related autoimmune related issues and possibly other inflammatory related issues such toxic chemical exposure.
Anti-IFN-γ in the treatment of autoimmune skin diseases **
Psoriasis vulgaris
Alopecia areata
Vitiligo
Acne vulgaris
Seborrheic dermatitis
Rosacea
Epidermolysis bullosa – genetic disease, also may have Th-1 autoimmune mechanism
Other skin diseases - herpes simplex virus type 2 (HSV2)
International studies utilizing second generation antibodies demonstrated actual use **
Psoriasis Vulgaris Treatment
before and after anti-IFN-γ treatment
Alopecia Areata Treatment
before and after anti-IFN-γ treatment
** Communication from Simon Skurkovich, MD, PhD and Boris Skurkovich, MD, PhD
Monoclonal Antibodies directed against Anthrax
Antibiotics therapy for individuals not previously vaccinated is not sufficient for the anthrax treatment because the infected individuals die as a result of action of bacilli virulence factors, namely components of B. anthracis exotoxin. It is widely accepted that the successful countermeasures to bacterial challenge should combine antibiotics therapy and passive immunization either by polyclonal sera or recombinant monoclonal antibodies (mAb’s) to the components of bacilli exotoxin. Although there are several anti-PA mAb’s developed to date, the idea of finding efficient combination of anti-PA and anti-LF mAb’s for the combination treatment of B. anthracis infection remains very attractive. First, the mAb combination can mitigate the spontaneous circumvention of epitope binding site within PA and LF as a part of natural bacterial ability to evade the immune system protection mechanisns. Second, if a synergetic mAb combination is found, then the antibody dose effect might be higher than for the single-antibody administration. It means that the treatment dosage can be lowered. The NIAID “Expert Consultation on Monoclonal Antibodies for Anthrax” has recommended that researchers consider developing a cocktail of mAbs which target different regions of the anthrax lethal toxin (LeTx).
Over the last decade the BTC team has, through various government supported projects, developed monoclonal antibodies directed against Bacillus anthracis (BA) and Yersinia pestis for the urgent prophylaxis and therapy of exposed individuals. For BA, this included antibodies against protective antigen of anthrax exotoxin (PA) and lethal factor (LF). Chimeric mouse-human monoclonal antibodies were developed first. The humanized versions of the anti-PA and anti-LF monoclonal antibodies (mAb’s) were then developed and expressed in E.coli cells. The Kd evaluation showed that the affinity of the resulting humanized antibody fragments was practically equal to the parental chimeric antibody in the case of the anti-LF antibody. The reduction of antigen-binding activity to low nanomolar levels was observed in the case of the anti-PA antibody. Our team subsequently evaluated the synergistic effect of the PA and LF antibodies.
In mouse challenge studies, it was demonstrated that the chimeric and humanized full-size anti-PA and anti-LF antibodies demonstrate the same level of protection against endospore challenge as the parental monoclonal antibodies. All of the biologicals obtained confer 50% protection level when administered to mice 3 h post-infection with B. anthracis spores. The neutralization of anthrax toxins was achieved as a result of synergistic action of the anti-PA and anti-LF antibodies.
The comparison of biological activity of human anti-PA and anti-LF mAb’s was described in the work of Albrect et. al. It was shown that the i.p. administration of either anti-PA or anti-LF 2.5 h before the endospore challenge resulted in 100% mice survival. Efficacy of antibody combination on mice protection against B. anthracis challenge has not been tested. The comparison of average life span of mice without protection (negative control) in the present study (2.8 days) and in (3.6 days) indicates that the assay performed here was carried out under more harsh conditions for the mice survival using different B. anthracis spores (Cenkovsky strain). Also, we administered antibody at 3 h and 24 h post-infection. The prophylactic model used by Albrecht et. al. is a totally different passive immunization regime compared to the post-challenge model. In the pre-challenge regimen, the immune system of mice is not damaged by toxin action, the levels of LeTx in circulation are lower, and, presumably, the blood titers of bacilli never reach the levels comparable to 3 h and 24 h levels in the case of post-challenge immunization. Supporting this assumption, there is an evidence that mAb’s can function in opsonic to clear vegetative bacilli. The 24 h post-challenge passive immunization protocol is a model of animal treatment under condition of severe damage to the immune system caused by extensive bacteremia and toxemia. Taking into account the average life span of mice in the control group (2.8 days), at 24 h post-infection some animals are close to pre-mortal state. Under such conditions PA272-LF58 mAb combination confers 50% survival rate that makes it the very promising antibody synergetic pair to be used for the development of therapeutic humanized mAb’s.
PA 272 and LF 58 Demonstrate Synergistic Effect
| Animal's Groups | Number of Dead Animals | Average Life-time | Cured Animals (%) |
|---|---|---|---|
| Control (anti F1) 3h* | 10/10 | 2,8 | 0,0 |
| Control (anti F1) 24h | 10/10 | 2,7 | 0,0 |
| LFA27 3h | 7/10 | 3,0 | 30,0 |
| LFA27 24h | 8/10 | 3,5 | 20,0 |
| LF58 3h | 9/10 | 4,0 | 10,0 |
| LF58 24h | 10/10 | 3,3 | 0,0 |
| PA241 3h | 9/10 | 4,0 | 10,0 |
| PA241 24h | 9/10 | 3,6 | 10,0 |
| PA272 3h | 7/10 | 4,4 | 30,0 |
| PA272 24h | 8/10 | 4,0 | 20,0 |
| PA241 + LFA27 3h | 7/10 | 4,4 | 30,0 |
| PA241 + LFA27 24h | 9/10 | 4,3 | 10,0 |
| PA241 + LFA58 3h | 9/10 | 4,0 | 10,0 |
| PA241 + LFA58 24h | 9/10 | 4,3 | 10,0 |
| PA272 + LFA27 3h | 5/10 | 4,2 | 50,0 |
| PA272 + LFA27 24h | 6/10 | 5,0 | 40,0 |
| PA272 + LFA58 3h | 4/10 | 5,5 | 60,0 |
| PA272 + LFA58 24h | 5/10 | 5,6 | 50,0 |
These results support the use of synergistic humanized antibodies for subsequent pre-clinical and clinical tests as drug candidates for the prophylaxis and treatment of B. anthracis - infected individuals.